Leadership
- Prof. Dr. Josef Käs, University of Lepizig
- Prof. Dr. Stefan Luther, Max Planck Institute for Dynamics and Self-Organization, Göttinen
- Dr. Alexander Schlemmer, Max Planck Institute for Dynamics and Self-Organization, Göttingen
One major topic within this domain is Physics of Cancer (Käs) which focuses on cell and tissue mechanics during tumor progression. From a physics perspective it seems to be highly unlikely that cells can squeeze through environments such as a cell cluster with no free space available. How cells move in dense environments is one of tissue biology’s most amazing enigmas connected to mechanics. The internal cytoskeletal mechanisms that permit cells to move have been intensely studied. However, this is only part of the answer. Physics of Cancer provides universal, quantitative criteria depending on the mechanical properties of a cell and its microenvironment when a cell can move or is jammed. In cell clusters at volume fraction one, there is no free space to pass each other. Cell jamming can be described as a kind of yield stress behavior. There is a mechanical threshold above which cells unjam and start to move. This threshold is determined by the balance of motile forces (protrusion and contractility), traction (i.e. adhesion) and cell’s viscoelastic resistance as well as cortical tension. Theoretical physics proposes that cells unjam when they can change from a round to an elongated shape at a specific aspect ratio. This may provide a simple yet powerful criterion to identify motile cells in tissues as well as tissue areas that behave as a fluid. In simple words, cell shape may determine if cells can move or not and in which tissues behave as a fluid or solid, respectively.
 Machine learning-based analysis will transform cancer diagnostics by exploiting three key advantages: (i) it uses conventional histological slides and does therefore not require any drastic changes to the standard diagnostic protocols, (ii) it is a quantitative, objective approach applicable across entities and tissues, and (iii) it allows aggressiveness to be predicted individually, even before metastatic tumors are formed. For a retrospective study 20,000 breast cancer cases are to be transferred into an integrated tumor database, which combines shape data with clinical data, data from classical histomorphology and immunohistochemical staining, as well as with gene expression profiles and molecular assays since an AI-based diagnosis fully unfolds only when all available complementary facts are used. We will develop detailed work flows ensuring a FAIR database buildup. Our task areas work towards an establishment of general canonical workflow models for cancer diagnostics within a clinical environment. In order to implement maximal flexibility, we will employ the concept of nanopublications which will allow to combine datasets at will within the present study and others, either adding existing data sets to make them fair or conceptualizing new studies. This is a challenging endeavor where we need to focus on specific aspects. It is a grand collaboration scheme where all task areas and domains work together to achieve our common goal.
Machine learning-based analysis will transform cancer diagnostics by exploiting three key advantages: (i) it uses conventional histological slides and does therefore not require any drastic changes to the standard diagnostic protocols, (ii) it is a quantitative, objective approach applicable across entities and tissues, and (iii) it allows aggressiveness to be predicted individually, even before metastatic tumors are formed. For a retrospective study 20,000 breast cancer cases are to be transferred into an integrated tumor database, which combines shape data with clinical data, data from classical histomorphology and immunohistochemical staining, as well as with gene expression profiles and molecular assays since an AI-based diagnosis fully unfolds only when all available complementary facts are used. We will develop detailed work flows ensuring a FAIR database buildup. Our task areas work towards an establishment of general canonical workflow models for cancer diagnostics within a clinical environment. In order to implement maximal flexibility, we will employ the concept of nanopublications which will allow to combine datasets at will within the present study and others, either adding existing data sets to make them fair or conceptualizing new studies. This is a challenging endeavor where we need to focus on specific aspects. It is a grand collaboration scheme where all task areas and domains work together to achieve our common goal.
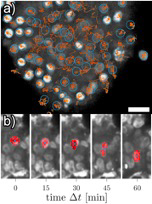
Fig 1 a) Cell tracks of SiR-DNA stained nuclei in a vital mammary carcinoma explant. The tumor shows areas of motile cells (middle) and jammed cells (border surrounding the center). The scale bar corresponds to 20 µm. b) Nucleus of a cancer cell that squeezes through ta dense micro-environment.
Another topic in the domain biomedical physics is the Dynamics of Heart Disease (Luther & Schlemmer). During normal rhythm, electrical excitation waves propagate through the heart muscle, resulting in coherent mechanical contraction and efficient blood pumping. In ventricular fibrillation (VF), however, complex spatio-temporal excitation patterns lead to fibrillatory contraction and loss of pumping efficiency. VF is immediately life-threatening and leads to approximately 100,000 sudden cardiac deaths per year in Germany alone. The mechanisms underlying the development and control of life-threatening arrhythmias are not well understood. For a lack of a better strategy, high-energy electrical shocks currently remain the only clinical option for terminating ventricular fibrillation. However, high-energy electric shocks have significant side effects, including excruciating pain, tissue damage, and worsening prognosis, indicating a substantial unmet medical need. Cardiac arrhythmias are examples of so-called “dynamic diseases.” Concepts and methods from the physics of complex systems are opening up new possibilities for diagnosis and therapy.
Our research combines the development of 4D cardiac imaging of the heart with unprecedented spatiotemporal resolution with data-driven modeling of electrophysiological and mechanistic processes towards new therapeutic methods for gentle arrhythmia control. In this context, methods of machine learning play an increasingly important role. The translation of basic and preclinical research into clinical application is one of the central challenges of quantitative life sciences. For this purpose, technologies have been developed to facilitate the management of large, heterogeneous and distributed data in interdisciplinary research networks.
Use cases
- Biomed01 Prof. Dr. Stefan Luther
- Biomed02 Prof. Dr. Josef Käs
- Biomed03 Prof. Dr. Matthias Günther
previous domain (Quantum Information and Artificial Intelligence) | domain list | next domain (Transdisciplinary Physics)